Our offer
Borosilicate glass
Borosilicate glass has been widely used due to the high chemical resistance and high resistance to temperature changes (thermal shock resistant). The level of boron oxide in the batch (the mixture of all raw materials, which are used to prepared glass) has a lasting effect on both the behavior of the melting glass, and on all other of its properties (including resistance to chemicals). Due to the possibility of differentiating the chemical composition (including the addition of metal oxides), the borosilicate glass form an extensive range of materials. We offer the following types of borosilicate glass: 3.3 borosilicate glass, borosilicate glass 4.3, Suprax 8488 and Pyrex, Boronorm, which selected properties are described below.
The typical hardness of borosilicate glass is 5.5 Mohs, Knopp 470, Vickers 580.
We offer the following types of borosilicate glass: 3.3 borosilicate glass, borosilicate glass 4.3, Suprax 8488 and Pyrex, Boronorm, which selected properties are described below.
Borosilicate glass 3.3 - DIN 7080
This type of glass is resistant to aggressive chemicals, has a high percentage of silica and large boron oxide dopant. It can be milled, drilled, grounded and toughened. Its low coefficient of thermal expansion, high thermal shock resistance and ability to work at temperatures up to 450 ° C for a long period of time, make this type of glass particularly suitable for use in stable temperature conditions. So borosilicate glass 3.3 is also called heat resistance glass.
Is suitable for operation at low temperatures. Can withstand the temperature to about -196 °C (is suitable for use in contact with liquid nitrogen). During thawing ensure that the temperature difference does not exceed 100 K. In general is recommended for use down to -70 °C.
This glass is extremely resistant to water, alkalis, acids and organic substances.
Borosilicate glass 4.3
To work in an environment of steam and hydrostatic applications we offer borosilicate glass 4.3. Resistance to chemicals and thermal expansion permit the use of a high level of hardening, so that the glass are characterized by high resistance to thermal shock. Is suitable for operation at low temperatures. Can withstand the temperature to about -196 °C (is suitable for use in contact with liquid nitrogen). During thawing ensure that the temperature difference does not exceed 100 K. In general is recommended for use down to -70 °C.
Borosilicate glass 3.3 - DIN 7080
Composition
| SiO2 | 80 % |
| B2O3 | 13 % |
| Na2O | 4 % |
| Al2O3 | 2 % |
| K2O | 1 % |
Standard thicknesses and tolerances
| Thickness | Tolerance | Thickness | Tolerance |
|---|---|---|---|
| 0,70 mm | ±0,1 | 7,5 mm | ±0,3 |
| 1,10 mm | ±0,1 | 8,0 mm | ±0,3 |
| 1,75 mm | ±0,2 | 9,0 mm | ±0,3 |
| 2,00 mm | ±0,2 | 13,0 mm | ±0,5 |
| 2,25 mm | ±0,2 | 15,0 mm | ±0,5 |
| 2,75 mm | ±0,2 | 16,0 mm | ±0,5 |
| 3,30 mm | ±0,2 | 17,0 mm | ±0,5 |
| 5,00 mm | ±0,2 | 18,0 mm | ±0,5 |
| 5,50 mm | ±0,2 | 19,0 mm | ±0,5 |
| 6,50 mm | ±0,2 | 21,0 mm | ±0,7 |
Properties:
| Density (@ 20 °C) | 2 230 kg/m3 |
| Bending strength | 160 N/mm2 |
| Surface compressive stress | 100 N/mm2 |
| Young’s modulus | 64 GPa |
| Poisson’s ratio | 0,2 |
| Hardness | 5.5 Mohs, (470 Knopp, 580 Vickers) |
| Thermal conductivity | 1,2 W/(m K) |
| Specific Heat | 0,83 kJ/(kg K) |
| Coefficient of linear expansion | 3,3 ±0,1 * 10 -6 °C |
| Index of refraction (@ 380 - 780 nm) | 1,48 |
| Softening point | 815 °C |
| Annealing Point | 560 °C |
| Max. working temperature: | |
| Non-tempered glass | |
| - long term | 450 °C |
| - temporary(< 10h) | 500 °C |
| Tempered glass | |
| - long term | 280 °C |
| - temporary(< 10h) | 500 °C |
Chemical properties:
Hydrolytic Resistance
Acc. ISO 719 (w 98 °C): class HGB 1
Acc. ISO 720 (w 121 °C): class HGA 1
Alkali resistance
Acc. DIN 52 322 (ISO 695): class A2
Acid resistance
Acc. DIN 12 116: class 1
Electrical properties
Volume resistance
at 25°C = 6.6 x 1013 Ω cm
at 300°C = 1.4 x 106 Ω cm
Dielectric properties
| Electric Volume Resistivity | 8,6 x 1013 Ωcm | (at 25 °C) | |
| 1.4 x 106 Ωcm | (at 300 °C) | ||
| Dielectric dissipation fraction | 38 10-4 | (at 1 MHz, 20 °C) | |
| Dielectric constant εr | 4.6 | (at 1 MHz, 20 °C) |
Optical properties
| Index of Refraction |
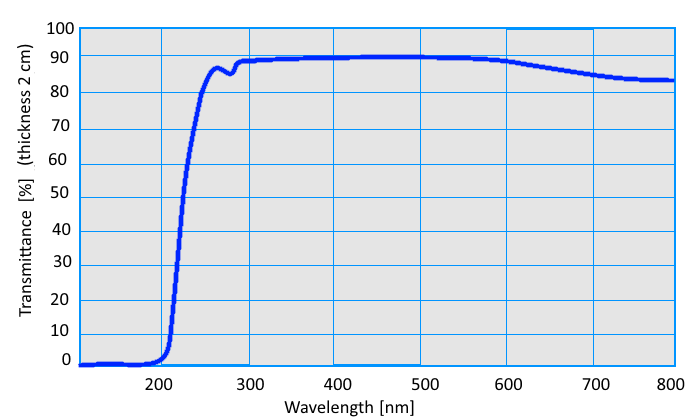
Spectral Transmission |
| λ = 587,6 nm nD = 1,4724 λ = 480,0 nm nF = 1,4782 λ = 546,0 nm nE = 1,4740 λ = 644,0 nm nC = 1,4701 |
 |
Borosilicate glass 4.3
| SiO2 | 78 % |
| B2O3 | 10% |
| Na2O | 7 % |
| Al2O3 | 3% |
| ZrO2 | 2 % |
Properties:
| Density (@ 25 °C) | 2 280 kg/m3 |
| Flexural strength | 25 MPa |
| Modulus of elasticity (Young’s) | 67 GPa |
| Poisson’s ratio | 0,20 |
| Thermal conductivity (@ 90 °C) | 1,2 W/(m K) |
| Specific heat | 0,83 kJ/(kg K) |
| Coefficient of linear expansion (@ 20 °C - 300 °C) | 4,3 * 10 -6 °C |
| Index of Refraction (λ=587,6 nm) | 1,484 |
| Softening point | 810 °C |
| Annealing point | 580 °C |
| Glass temperature for density dPas |
1013,0 560 °C |
| Working temperature: | |
| - maximum | 500 °C |
| - in a heavy duty conditions | 280 °C |
Chemical properties
Hydrolytic Resistance
Acc. ISO 719 (@ 98 °C): class HGB 1
Acc. ISO 720 (@ 121 °C): class HGA 1
Alkali resistance
Acc. DIN 52 322 (acc. ISO 695): class A2
Acid resistance
Acc. DIN 1776: class 1
Electrical properties
Volume resistance
@ 25°C = 6.6 x 1013 Ω cm
@ 300°C = 1.4 x 106 Ω cm
Dielectric properties
@ 25° C and 1 MHz:
Dielectric constant εr=4,6
Dielectric loss factor tgδ =1,4x10-2
Optical properties
| Index of Refraction | Spectral Transmission |
| λ = 587,6 nm nD = 1,4816 λ = 480,0 nm nF = 1,4869 λ = 546,0 nm nE = 1,4831 λ = 644,0 nm nC = 1,4802 |
 |
While every attempt has been made to verify the source of the information, no responsibility is accepted for accuracy of data.





